Abstract
Background: The International Prognostic Scoring System-Revised (IPSS-R) is one gold standard for myelodysplastic syndrome (MDS) risk stratification. An accurate estimate of prognosis allows clinicians to make appropriate management decisions. The IPSS-R intermediate-risk (int-risk) group however, shows marked heterogeneity with respect to disease outcomes, and contributes to uncertainty about patient management. A more refined yet simple sequential scoring model is therefore proposed to improve the classification of int-risk patients to allow for appropriate therapeutic decisions.
Methods: A retrospective patient-centered analysis was conducted at MD Anderson Cancer Center on patients diagnosed with primary MDS between 2000 and 2015. All patients were scored according to the IPSS-R criteria and int-risk patients were included. The primary outcome was overall survival (OS), and covariate characteristics included: hemoglobin, ferritin, white blood cell (WBC) count, platelet count, absolute neutrophil count (ANC), percentage of peripheral blood (PB) and bone marrow (BM) blasts at diagnosis, number of cytopenias, performance status, RBC and platelet transfusion history, and type of treatment received. Survival differences between groups were compared and univariate Cox proportional hazards regressionwas used to evaluate association between each covariate and OS. Classification and regression tree (CART) analysis was used to find the best cut-off points to differentiate OS for some continuous variables. CART analysis for failure time data used the Martingale residuals of a Cox model to approximate chi-square values for all possible cut points. A multivariate Cox proportional hazards model was then fitted for OS by including all statistically significant covariates from univariate Cox models. A backward stepwise selection was performed using a threshold significance of 0.05 for covariates to stay in the final model. Internal validation testing of the final stratification score was performed using the bootstrap method, and external validation was performed using an independent external cohort from Cleveland Clinic.
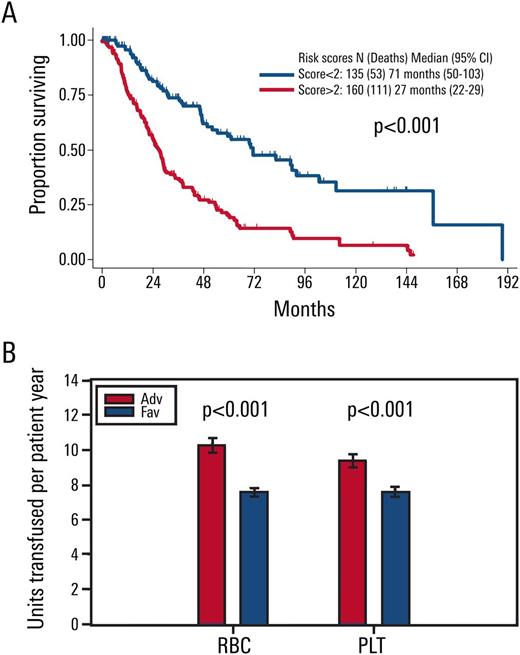
Results: Out of 3167 patients, a total of 298 were identified with IPSS-R int-risk MDS and retrospectively analyzed in this study. Age of 66 years or greater (2 points), peripheral blood blasts of 2% or more (1 point), and history of RBC transfusion (1 point) were significantly associated with inferior survivaland were included in the final OS prognostic model. The score divided MDS patients who have IPSS-R intermediate-risk into two prognostic risk groups: an int-favorable group with a score of 0-1, and an int-adverse group with a score of 2-4. Overall survival was significantly different between the two risk groups of patients, with median survival of approximately 6 and 2 years for int-favorable and int-adverse, respectively (p <0.001, Figure 1A). Transfusions per patient year were significantly higher in int-adverse patients for both red blood cells and platelets (p<0.001, Figure 1B). The stratification prognostic score was robust in the classification of patients with IPSS-R intermediate-risk myelodysplastic syndrome by internal validation, and analysis of 111 IPSS-R int-risk MDS patients from a separate institution demonstrated significantly different OS between int-favorable and int-adverse patients, with median survival of approximately 4 and 2 years respectively (p=0.04).
Conclusions: Sequential prognostication provides a clinically practical framework to aid clinicians in determining appropriate treatment approaches for the heterogeneous group of int-risk MDS patients.
Jabbour: Bristol-Myers Squibb: Consultancy. Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau. Steensma: Pfizer: Consultancy; Incyte: Equity Ownership; Janssen: Consultancy, Research Funding; H3 Biosciences: Consultancy; Celgene: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Onconova: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Roboz: Cellectis: Research Funding; AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace: Consultancy. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Kantarjian: ARIAD: Research Funding; Pfizer: Research Funding; Delta-Fly Pharma: Research Funding; Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal